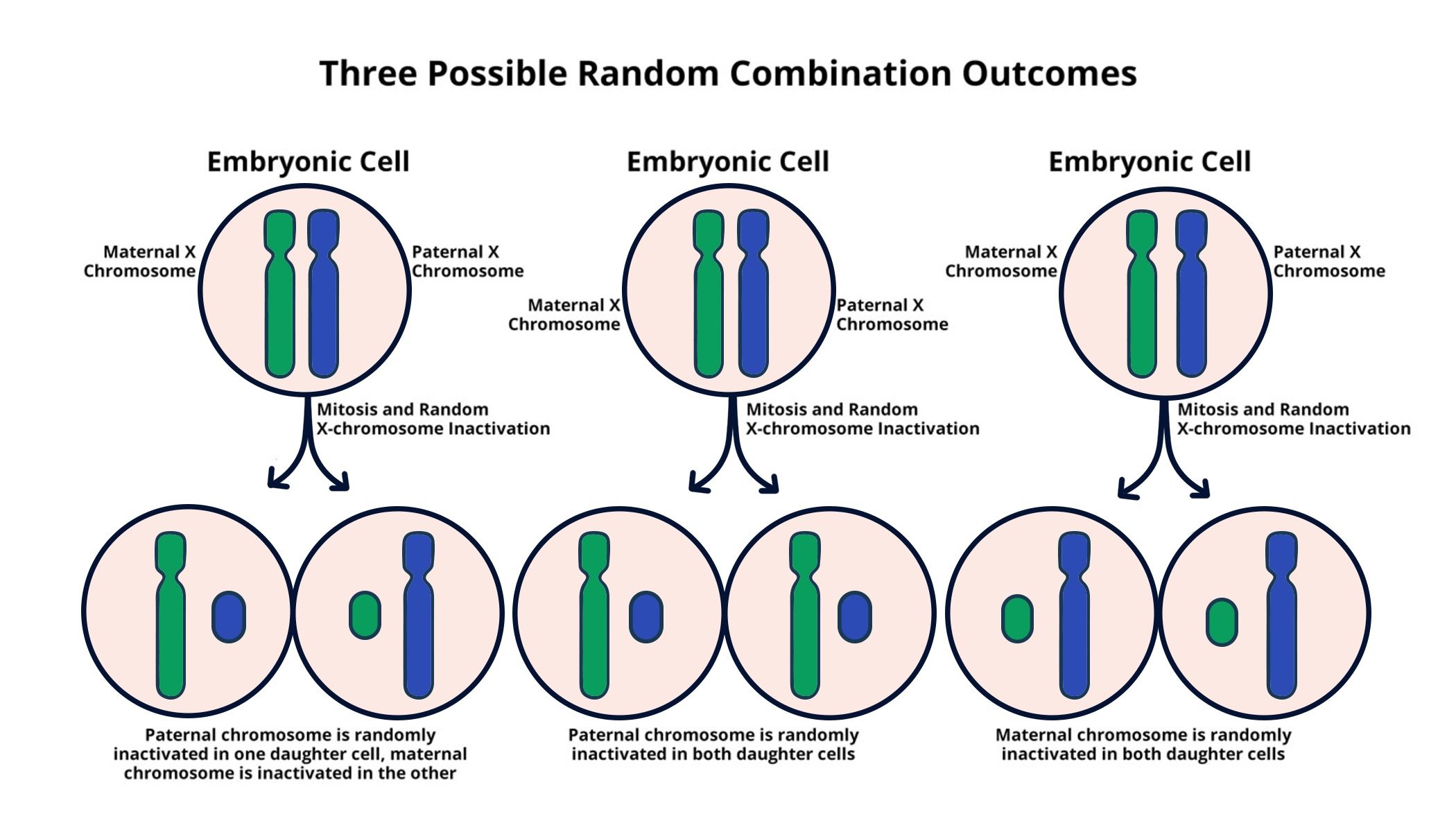
X chromosome inactivation is a fascinating biological process that has significant implications for understanding genetic disorders. In females, with their two X chromosomes, one is inactivated to maintain a balance of gene expression, ensuring that they do not have an excess of gene products compared to males, who possess only one X. This mechanism has attracted the attention of researchers like Jeannie Lee, whose groundbreaking studies could pave the way for innovative therapies targeting conditions like Fragile X Syndrome and Rett Syndrome. The chromosomal breakthroughs stemming from this research not only unravel the complexities of X inactivation but also unveil potential therapeutic strategies for those affected by debilitating genetic disorders. By exploring the gelatinous substance that encapsulates chromosomes, Lee’s work highlights the intricate dance of molecules that may lead to new treatment avenues, offering hope for a brighter future for many.
The process of X chromosome inactivation, or lyonization, is essential to balance gene expression between genders. This intriguing phenomenon, primarily studied in females, allows one of the two X chromosomes to become silent while still preserving the functionality of the other. Researching the mechanisms underlying this silencing, especially through the lens of Jeannie Lee’s insightful contributions, is shedding light on potential interventions for genetic disorders, including Fragile X and Rett Syndromes. By advancing our understanding of how chromosomal behavior can be manipulated, we are moving closer to developing effective treatments that could greatly improve patients’ lives. The exciting revelations from this field not only enhance our grasp of chromosomal biology but also suggest a promising future for innovative therapies.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that ensures gene dosage balance in females. As females possess two X chromosomes, the inactivation of one copy is essential to prevent a double expression of genes that may lead to cellular dysfunction. The discovery of XCI has been a cornerstone in genetics, influencing our understanding of various genetic disorders, including Fragile X and Rett syndromes. Researchers have long sought to comprehend the mechanisms governing XCI, and significant strides have been made thanks to pivotal studies, including recent findings by Jeannie Lee that shed light on the intricate orchestration of this biological silencing.
In her pioneering research, Jeannie Lee has elucidated the role of a gelatinous, Jell-O-like substance surrounding chromosomes, which facilitates X chromosome inactivation. The XCI process relies heavily on a specific RNA molecule called Xist, which initiates changes in the surrounding material. This modification helps to create a more flexible environment, allowing other crucial molecules to access and suppress gene activity on the inactivated X chromosome. This discovery not only advances our understanding of basic genetic principles but also opens new avenues for potential therapies for X-linked genetic disorders.
Innovative Therapies Derived from Chromosomal Breakthroughs
The breakthroughs in understanding X chromosome inactivation have significant clinical implications, particularly for individuals affected by Fragile X Syndrome and Rett Syndrome. By targeting the silencing mechanism, researchers like Jeannie Lee are exploring ways to ‘unsilence’ the genes associated with these conditions, thus providing hope for effective treatments. Fragile X Syndrome is linked to intellectual disabilities caused by mutations on the X chromosome, while Rett Syndrome is a devastating neurodevelopmental disorder. Both conditions are intricately tied to the gene regulation processes that occur on the X chromosome.
Initially, the research focused on understanding how inactivation occurred, but with new insights, the focus has shifted towards therapeutic applications. The ability to manipulate the X chromosome’s inactivation state could lead to innovative treatments that rectify gene expression without altering healthy gene function. As clinical trials loom on the horizon, the implications of this work are groundbreaking. If successful, these therapies could not only alleviate the symptoms of Fragile X and Rett Syndromes but could also push the boundaries of genetic disorder treatments more broadly.
The Role of Jeannie Lee’s Research in Chromosomal Advances
Jeannie Lee’s research is at the forefront of exploration into chromosomal dynamics, specifically focusing on the X chromosome’s unique challenges. By utilizing advanced techniques to unravel the complexities of X chromosome inactivation, her lab has opened up discussions around potential therapies targeting genetic disorders. This research not only highlights the intricacies of XCI but also emphasizes how a greater understanding of chromosomal behavior can lead to significant breakthroughs in the treatment of diseases tied to X-linked mutations.
Lee’s work exemplifies the power of collaboration and persistence in scientific endeavors. After 25 years of extensive research funded by the National Institutes of Health, the findings from her lab are now transitioning from theoretical understanding to practical applications in medicine. This journey from basic research to potential therapies reflects the significant impact that chromosomal studies can have on treating various genetic disorders, and serves as a beacon of hope for affected individuals and their families.
Implications of X Chromosome Inactivation for Male Patients
While the X chromosome inactivation process predominantly affects females, it does not entirely exclude male patients who are impacted by X-linked genetic disorders such as Fragile X Syndrome. Males possess only one X chromosome, but mutations on this chromosome can disrupt normal gene function, leading to significant health issues. Understanding the inactivation of the second X chromosome in females offers insights into gene regulation that may also be relevant for therapies in males. Essentially, strategies designed to reactivate inactivated genes could prove beneficial across genders, although they would need to be tailored to account for distinct biological differences.
Current research indicates that treatments developed from insights into X chromosome behavior may be advantageous for males carrying mutations linked to fragility in their single X chromosome. This approach could potentially enable the restoration of gene function similarly to that in females, where one healthy gene could compensate for a mutated counterpart. Thus, the implications of Lee’s research extend beyond traditional boundaries, suggesting potential therapeutic avenues for a broader spectrum of patients suffering from genetic disorders.
Transformative Effects of Novel Genetic Therapies
The pursuit of therapies based on chromosomal breakthroughs signifies a transformative shift in genetic medicine. As scientists like Jeannie Lee unveil the complexities of X chromosome inactivation, the potential to develop targeted treatments becomes increasingly tangible. By harnessing the discoveries regarding Xist and its impact on the surrounding jelly-like substance, researchers are in a position to devise strategies that could correct the gene dysfunctions seen in Fragile X and Rett Syndromes. Such advancements may revolutionize how we approach genetic therapies, shifting from symptomatic treatments to potential cures.
A critical aspect of these novel therapies lies in their capacity to modify gene expression with minimal disruption to the healthy genes on the X chromosome. The aim is to ‘unsilence’ the inactive X chromosome without adversely affecting the overall genetic framework of the patient. This delicate balance is central to developing effective therapeutic interventions that offer sustainable results with reduced side effects. As the research progresses, future clinical applications will likely continue to therapeutically exploit the intricate connections established in Lee’s work and others in the field.
Future Directions in X Chromosome Research
The future of X chromosome research looks promising, with Jeannie Lee and her team poised to lead the charge in unraveling further details about XCI and its aftermath. As understanding grows, so does the potential for innovative treatments aimed at genetic disorders closely associated with this chromosome. The continued exploration of how to manipulate X chromosome inactivation not only holds the promise of alleviating conditions like Fragile X and Rett Syndromes but might also provide insights into entirely new therapeutic modalities for other genetic disorders.
Moreover, as technology advances, researchers are likely to develop more refined methodologies for analyzing gene function and expression within the chromosomal context. This progress could pave the way for improved screening techniques to identify additional X-linked genetic disorders, potentially leading to early interventions that could mitigate symptoms before they manifest drastically. Establishing these cutting-edge strategies will be vital in shaping the next generation of genetic therapy development and enhancing the quality of life for those with genetic predispositions.
Recent Advances in Genetic Disease Therapies
Recent advances in genetics have illuminated new pathways for treating diseases, particularly those linked to the X chromosome. Jeannie Lee’s findings have sparked a wave of hope for those affected by disorders like Fragile X Syndrome, where understanding the intricacies of X chromosome inactivation is proving to yield potential solutions. These innovations emphasize the importance of chromosomal research in unlocking new treatment strategies focused on gene regulation and expression.
The ramifications of these advances are profound; they represent a shift towards precision medicine, where therapies can be tailored to the specific genetic profiles of patients. This personalization could drastically improve treatment outcomes for individuals with X-linked genetic disorders, as therapies become increasingly focused on rectifying the underlying causes rather than simply addressing the symptoms. As genetic research continues to evolve, the collaborative efforts of scientists and clinicians will be crucial in bringing these innovative therapies to fruition.
Understanding the Role of Chromosomal Structures in Gene Expression
The structural elements surrounding chromosomes play a formative role in regulating gene expression, a crucial aspect that Jeannie Lee’s research has highlighted. By unraveling the complexities of X chromosome inactivation, researchers have gained insight into how gene activity is not just determined by sequences of DNA but also by their chromosomal environment. The Jell-O-like substance surrounding chromosomes exemplifies how structural elements can influence which genes are expressed and which remain dormant.
This understanding extends beyond just the X chromosome and opens up inquiries into how chromosomal architecture contributes to disorders across the genome. As scientists delve deeper into the interactions between chromosomal structures and gene functionality, the potential for discovering novel therapeutic targets increases. Such knowledge could ultimately reshape how genetic disorders are approached, facilitating breakthroughs in treatments that harness the power of chromosomal behavior.
The Future of Gene Therapy in Treating Genetic Disorders
As we look ahead to the future of gene therapy, the implications of Jeannie Lee’s research on X chromosome inactivation could play a pivotal role. With the groundwork laid for understanding how to manipulate genetic expression, the potential to treat or even cure debilitating genetic disorders is within reach. The challenge lies in converting laboratory discoveries into effective clinical applications, which requires a concerted effort from researchers, clinicians, and regulators alike.
In the coming years, advancements in gene therapies aiming to rectify genetic mutations have the potential to revolutionize treatment landscapes for conditions like Fragile X Syndrome and Rett Syndrome. By continuously refining their approaches, researchers may develop strategies that not only address the disease symptoms but also restore normal gene function. Such innovation could redefine the course of genetic disorder treatment, allowing affected individuals to lead fuller, more active lives.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation is a process that occurs in female mammals where one of the two X chromosomes is silenced, ensuring that females do not have double the gene dosage compared to males. This phenomenon is crucial in preventing genetic disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Understanding this process can lead to potential therapies for these conditions by enabling the activation of the healthy X chromosome genes.
How does Jeannie Lee’s research contribute to our understanding of X chromosome inactivation?
Jeannie Lee’s research has greatly advanced our knowledge of X chromosome inactivation by uncovering how the Xist RNA molecule alters the properties of a gelatinous substance surrounding the X chromosome. Her studies reveal how this process can lead to potential therapies for genetic disorders like Fragile X and Rett Syndromes, as it opens avenues for therapeutically unsilencing the inactivated X chromosome.
What role does Xist RNA play in the X chromosome inactivation process?
Xist RNA is essential for X chromosome inactivation as it coats the chromosome and modifies the ‘Jell-O-like’ substance surrounding it. This interaction allows other molecules involved in the inactivation to penetratively engage with the chromosome, effectively leading to its silencing. The insights into Xist’s function are critical for developing therapies for conditions caused by X-linked mutations.
Can therapies developed from X chromosome inactivation research help treat Fragile X Syndrome?
Yes, therapies stemming from research on X chromosome inactivation aim to reactivate healthy genes on the inactivated X chromosome in individuals affected by Fragile X Syndrome. Strategies developed in Jeannie Lee’s lab show promise as they focus on restoring the function of mutated genes while minimizing impacts on healthy genes, thereby potentially alleviating the symptoms associated with this genetic disorder.
What therapeutic advancements can we expect from the research into X chromosome inactivation?
The advancements in the research of X chromosome inactivation may lead to the development of novel treatments for genetic disorders like Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s team is actively working on optimizing methods to unsilence inactivated X chromosomes, with the goal of transitioning to clinical trials within the next few years, promising new hope for patients affected by these conditions.
Why is X chromosome inactivation particularly relevant for conditions like Rett Syndrome?
X chromosome inactivation is especially relevant for conditions like Rett Syndrome because it highlights the potential to target the healthy but silenced genes present on the inactive X chromosome. Since Rett Syndrome is linked to mutations on the X chromosome, unlocking these genes could provide therapeutic options to restore normal function and mitigate the disorder’s effects.
What are the implications of unsilencing inactivated X chromosomes for male patients?
While males typically have only one X chromosome and do not undergo X chromosome inactivation, some therapeutic strategies derived from understanding this process may still benefit them. For instance, these strategies could address mutations that specifically affect genes on the X chromosome, such as those related to Fragile X Syndrome, leading to potential treatments even in male patients.
What challenges remain in the study of X chromosome inactivation related to genetic disorders?
Despite the breakthroughs in understanding X chromosome inactivation, challenges remain regarding the precise mechanisms of gene regulation and the selective unsilencing of mutated genes without affecting the healthy counterparts. Ongoing research is necessary to fully comprehend these dynamics and to translate basic science into effective clinical therapies for genetic disorders.
| Key Points | Details |
|---|---|
| The X Chromosome in Females vs Males | Females have two X chromosomes, but one is inactivated to prevent double gene expression. |
| Role of Xist RNA | Xist is produced by a gene on the X chromosome, modifying the surrounding ‘Jell-O’ substance to facilitate inactivation. |
| Importance of the Gelatinous Substance | The Jell-O-like material prevents chromosomes from tangling and assists in the inactivation process. |
| Therapeutic Implications | Research aims to ‘unsilence’ inactivated X chromosomes to treat disorders like Fragile X and Rett syndromes. |
| Future Directions | The next steps involve optimizing treatment approaches and moving towards clinical trials. |
Summary
X chromosome inactivation is a critical process by which females silence one of their two X chromosomes to maintain gene dosage balance with males. This complex biological mechanism, involving the RNA molecule Xist and a gelatinous substance surrounding chromosomes, has significant implications for treating genetic disorders linked to the X chromosome, including Fragile X and Rett syndromes. Understanding and potentially manipulating this process opens the door for innovative therapeutic strategies in the future.